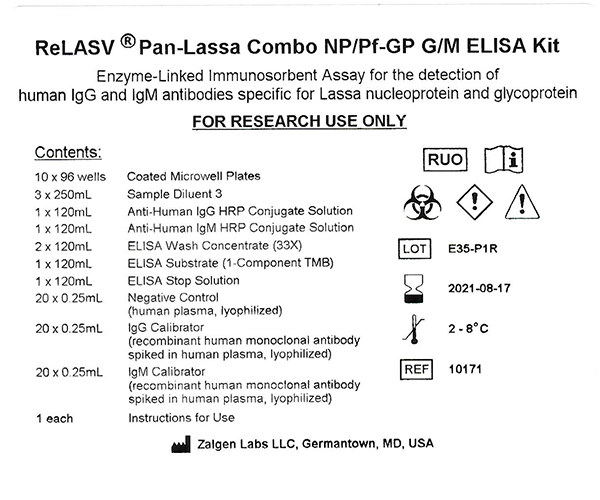
This Enzyme-linked Immunosorbent Assay (ELISA) detects human IgG or IgM antibody specific for LASV NP and GP antigens. Diluted plasma or serum samples, Calibrator, Positive Control, and Negative Control are incubated in microwells coated with a mixture of recombinant LASV NP and Prefusion GP antigens. Incubation allows the LASV-specific antibodies present in the samples to react with the immobilized antigen mixture. After the removal of unbound plasma or serum proteins with the PBS-Tween wash, the anti-human IgG (or IgM) – horseradish peroxidase (HRP) conjugate reagent is added to bind with the absorbed LASV-specific IgG (or IgM) antibody. Following another washing step, the bound enzyme-antibody conjugate is assayed by the addition of TMB substrate.
Color develops in the wells at an intensity proportional to the concentration of the LASV-specific IgG (or IgM) antibody in the sample. Substrate color development is stopped by addition of the ELISA Stop Solution. Optical Density (O.D.) results are obtained by reading the absorbance at 450nm (minus 620 – 650nm reference) using an ELISA plate reader. It is recommended that the user establish a cut-off for the study population using LASV sero-negative samples. It is also recommended that well characterized, IgG and IgM positive, convalescent LF samples from the study population be included in each assay as additional reference samples.
Click here for package insert for Pan-Lassa Combo NP-PfGP GM ELISA Kit (RUO)
Click here for SDS for Pan-Lassa Combo NP-PFGP GM ELISA Kit (RUO)