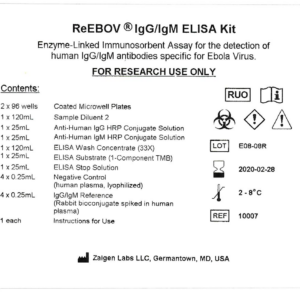
This test is a direct ELISA detecting Human IgG and/or IgM antibody specific for Ebola virus (EBOV) viral matrix protein 40 (VP40). Diluted samples, Reference, and Negative Control are incubated in microwells coated with recombinant EBOV VP40 antigen. Incubation allows the anti-EBOV antibody present in the samples to react with the immobilized antigen. After the removal of unbound serum or plasma proteins by washing, anti-human IgG (or IgM) antibodies, labeled with horseradish peroxidase (HRP), are added, forming complexes with the bound IgG (or IgM) anti-EBOV antibody. Following another washing step, the bound enzyme-antibody conjugate is assayed by the addition of a solution containing tetramethylbenzidine (TMB) and hydrogen peroxide (H2O2) as the chromogenic substrate. Color develops in the wells at an intensity proportional to the serum concentration of IgG (or IgM) anti-EBOV antibody. Results are obtained by reading the OD (optical density or absorbance) of each well in a spectrophotometer. A Reference non-human bioconjugate and Negative serum control are provided. It is recommended that the user establish a cut-off for the study population using at least 100 sero-negative serums. It is also recommended that IgG and IgM positive convalescent Ebola serums from the study population be included in each test as an additional reference sample.
Ebola Diagnostic Kits, Glycoprotein, VHF Diagnostic Kits
ReEBOV® IgG/IgM ELISA Kit – RUO (10007) – Special Order
$2,000.00 – $10,000.00Price range: $2,000.00 through $10,000.00
Designation: Semi-quantitative detection of human IgG and IgM antibodies specific to Ebola VP40 antigen
Specificity: VP40
Applications: ELISA microplate
Storage: 2-8°C
Shipping: Refrigerated; blue ice packs
Ordering: Item 10007 is a special order product, and inventory may be limited. Contact Zalgen for current status and projected availability.
SKU: 10007
Categories: Ebola Diagnostic Kits, Glycoprotein, VHF Diagnostic Kits
Related products
-
Glycoprotein
ReLASV® Linked GP Lineage IV IgG ELISA Kit – RUO (10732) – Special Order
$1,800.00 – $9,000.00Price range: $1,800.00 through $9,000.00 Select options This product has multiple variants. The options may be chosen on the product page -
Glycoprotein
ReLASV® Pan-Lassa Prefusion GP IgM ELISA Kit – RUO (10144) – Special Order
$2,000.00 – $10,000.00Price range: $2,000.00 through $10,000.00 Select options This product has multiple variants. The options may be chosen on the product page -
Antigen
ReLASV® Pan-Lassa Antigen ELISA Kit – RUO (10003) – Special Order
$1,000.00 – $10,000.00Price range: $1,000.00 through $10,000.00 Select options This product has multiple variants. The options may be chosen on the product page -
Glycoprotein
ReLASV® Pan-Lassa Prefusion GP IgG/IgM ELISA Kit – RUO (10580)
$2,000.00 – $10,000.00Price range: $2,000.00 through $10,000.00 Select options This product has multiple variants. The options may be chosen on the product page