“Diagnostic testing has become indispensable for diagnosing and monitoring disease, for providing prognoses and for predicting treatment responses… These include traditional laboratory-based tests, with samples being sent to a central laboratory for analysis, and point-of-care tests, which can be performed near, or at, the point of patient care. Point-of-care testing can help optimize treatment decision-making, avoid referrals, improve the efficiency of care and decrease costs, especially in resource-constrained settings where laboratory infrastructure is weak.”
(Kosack, et al, Bulletin of the World Health Organization 2017; 95:639-645)
Diagnostic Test Kits
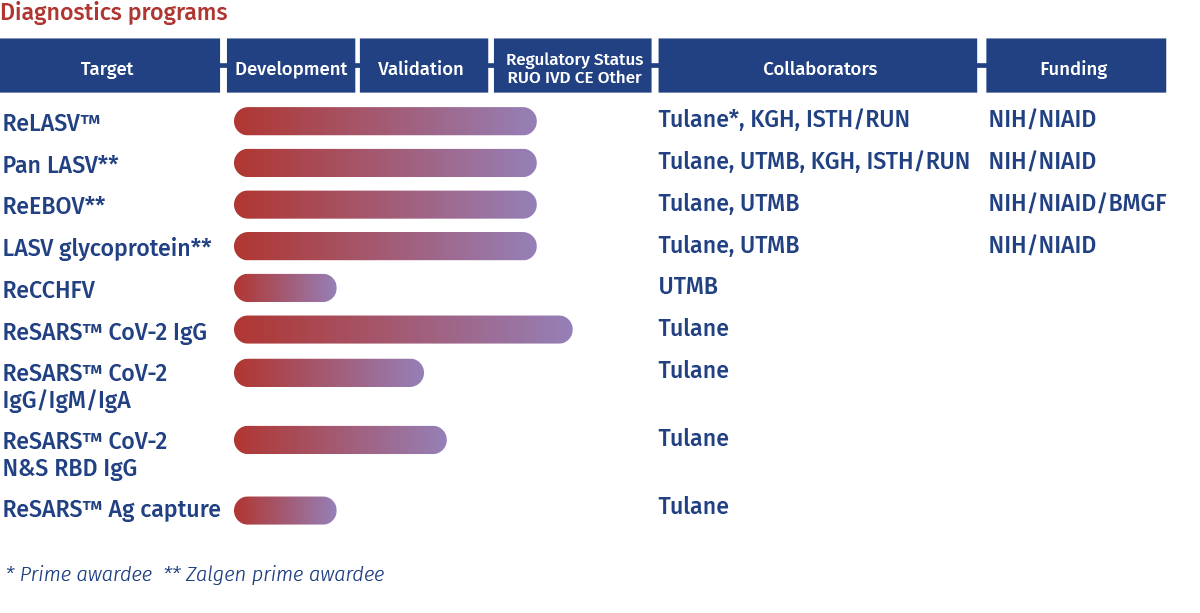
Zalgen already manufactures the most extensive line of immunodiagnostic test kits (Enzyme-Linked Immunosorbent Assay [ELISA] for laboratory use) and Rapid Immunodiagnostic Tests [RDT] for point-of-care testing or use in the laboratory) for viral hemorrhagic fevers including Lassa and Ebola. These diagnostic tests enable researchers and investigators to detect acute viremia (circulating antigen) and human convalescent immune responses (IgM and IgG).
For Lassa virus, Zalgen currently manufactures over 20 different diagnostic tests in both ELISA and RDT platforms. Products in the development pipeline include additional single lineage and Pan-Lassa products, improved delivery platforms and combination products to address needs of researchers.
For Ebola virus, Zalgen currently manufactures 4 different diagnostic tests in ELISA and RDT format. Products in the development pipeline include improved formatted products, as well as products detecting additional filoviruses.

(L-R) Mr. Augustine Goba, Dr. Matt Boisen, Dr. Robert Garry. Testing samples from suspected Ebola patients with the ReEBOV Antigen Rapid Test, the first rapid Ebola product to receive Emergency Use Authorization by both the FDA and the WHO. Kenema Government Hospital, Kenema, Sierra Leone. (2015)
Critical Reagents
Zalgen designs and produces superior biological molecules critical for the development and commercialization of immunotherapeutics, vaccines, and reliable, rapid, and affordable diagnostic platforms. The company uses its proprietary expression platforms, including its patented mammalian cell-based biomanufacturing system, CHOLCelect, to deliver next generation biologicals to world health and biodefense settings.
Current critical reagent products for hemorrhagic fever viruses include recombinant antigens, human monoclonal antibodies (HuMAb) from survivors, monoclonal and polyclonal antibodies generated in multiple mammalian species, and recombinant virus-like particles (VLP) that incorporate custom combinations of arenaviral proteins. New products in the development pipeline include reagents, antibodies, and diagnostics for additional emerging hemorrhagic fevers, including Crimean Congo Hemorrhagic Fever virus (CCHFV).
